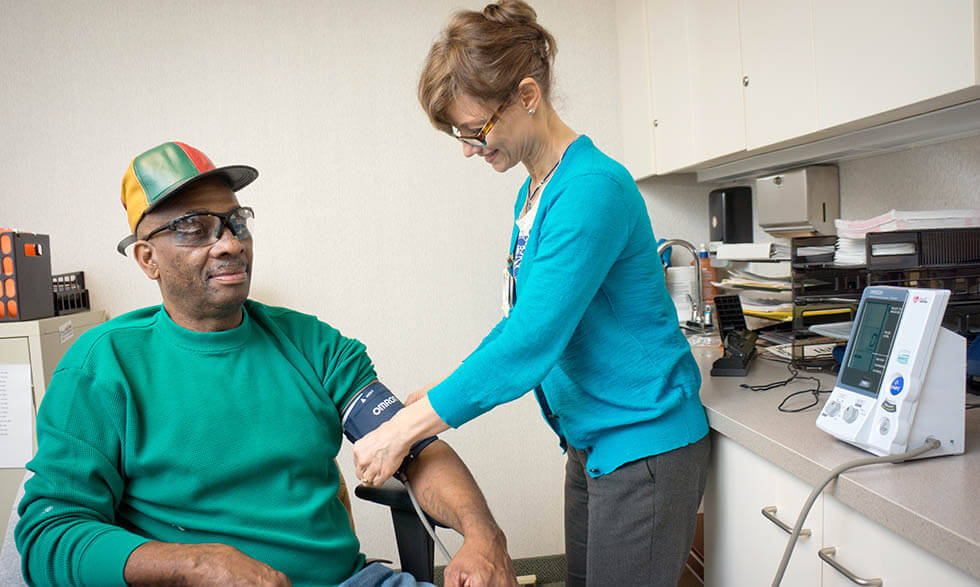
Do you take a statin for high cholesterol? Does ibuprofen help you with aches and pains? These medicines were once studied in a clinical trial. Now, millions of people take them every day.
Clinical trials or studies happen when medicines or tools that have been tested for safety in a lab are ready to test in people. Some people participate in clinical trials because none of the standard (approved) treatment options have worked, or they are unable to tolerate certain side effects. For others, it's an opportunity to help researchers find new ways to prevent, detect, or treat diseases.
A number of clinical trials take place right at the National Institutes of Health (NIH) through the NIH Clinical Center, the nation's largest research hospital.
Clinical trials evaluate:
- New ways to find a disease early, sometimes before there are symptoms
- How to safely use a treatment or different ways to use current treatment more effectively
- New approaches to surgery and new medical devices
- Vaccines and lifestyle changes that can help prevent a disease
- Improvements to the comfort and quality of life for people with short- or long-term illnesses
How do clinical trials work?
The idea for a clinical trial often starts in a lab, where scientists identify a promising potential treatment for development and conduct experiments to gather information to find out if it could cause serious harm. Following this research and testing, the Food and Drug Administration (FDA) may then give approval for testing in humans in a clinical trial.
Clinical trials happen in a series of four steps called "phases." Each has a different purpose and helps researchers answer different questions about treatments, risks, and side effects.
- Phase I: Researchers study a new treatment in a small group of people (20 to 80) to identify the correct dose and effect on the body.
- Phase II: The treatment is tested in a larger group of people (100 to 300) to confirm it works effectively and further study its safety.
- Phase III: The treatment is tested in an even larger group (1,000 to 3,000) to further monitor any side effects or compare it with similar treatments.
- Phase IV: After a treatment is approved by the FDA, it's made available to the public. Researchers continue to track its safety in the general population. They will collect information about the treatment's benefits and the best ways to use it.
Who is involved?
Clinical trials typically have a research team that includes doctors, nurses, or other health care professionals. Trials will also have a plan designed to answer specific research questions and information for a person to understand the risks and potential benefits. Each trial has certain requirements, known as eligibility criteria, for who can participate. Some may want healthy participants. Others may need volunteers with a certain disease. Adults, children, and people of different ethnic and racial backgrounds are all encouraged to participate if they qualify.
Finding the right trial for you
You may volunteer to participate in a clinical trial, or you may be recruited. You should ask about the purpose of the trial and who has approved it. Other good questions to ask include who will fund the study, what you'll need to do, and how long it will last. Before participating in a study, talk to your health care provider or other trusted advisors and learn about the risks and potential benefits.
Interested in joining a clinical trial? Visit the National Library of Medicine's (NLM) ClinicalTrials.gov website. ClinicalTrials.gov is a registry and results information database of clinical research studies sponsored or funded by public and private organizations around the world. The listings are provided by the study sponsor and investigators and have not been evaluated by the U.S. government. You can search by disease or condition, medicine or treatment, location, and more.